Ther

profile
CHRISTINA GUNAWAN(06);
PLMGS(S); 3B1
30july93
Photography club
about
THERMAL PHYSICS; Chapter 7, 8 & 9 of the physics textbook.(i like pictures, so you know what to except >:D)
archives
Pattern: tillyness
|
Ther
 profile
CHRISTINA GUNAWAN(06); PLMGS(S); 3B1 30july93 Photography club about
THERMAL PHYSICS; Chapter 7, 8 & 9 of the physics textbook.(i like pictures, so you know what to except >:D) archives
Pattern: tillyness |
mal♥
Amanda Liew (01) Brenda (02) Lee Huang (03) Chara (04) Cherlyn (04) Christina :D :D :D(06) Xin Yan (07) Wen Shi (08) Clarice (09) Jolene Ek (10) Germaine (11) Clara (12) Isabel (13) Joann (14) Leah (15) Amanda Lim (16) Zi Ai (17) Maureen (18) Melissa (19) Mii Wei (20) Moy Kit (21) Li Ying (22) Neo yun (23) Wan Leng(24) Samantha Ang (25) En Yin (26) Shenna (27) Sheryl (28) Shobana (29) Stephanie (30) Jesslyn (31) Valerie (32) Yu Xian (33) Ying Ching (34) Qing Hui (35) Cassandra (36) Vanessar (37) Xiao Wei (38) Jia Yee (39) Kai Jing (40) Joaquim (41) |
Physics
Saturday, September 6, 2008
went around youtube searching for some physics videos, but found this!SUPERCOOOOOLING! :D omgggg, i think its so cooooooooooooooooool. hahas, ''Supercooling is the process of chilling a liquid below its freezing point, without it becoming solid.'' - Wikipedia Thursday, September 4, 2008
Chapter 9 - Thermal Properties of MatterWhat is internal energy? o.O - Internal energy is particles in a solid vibrating about in a fixed position & are held together by strong interatomic/intermolecular bonds. -Internal energy comprises of 2 components - kinetic energy & potential energy Kinetic Energy - Is the vibration of the particles - Directly related to temperature - The higher the temperature, the more vigorous the vibration of the particles - For liquid & gas, kinetic energy is due to their movement - One example is the mircowave oven :D  thats why your ice cold food get pipping hot in few seconds! :D thats why your ice cold food get pipping hot in few seconds! :DPotential Energy - Is the stretching & compressing of intermolecular bonds as the particle vibrates - Amt of potential energy stored in the bonds depends on the force between the particles & how far apart they are. - One example is the slinky :D  Melting - Is when a solid changes to a liquid upon heating.  must appreciate this graph, okay! cause i took a million years to draw! :D but any mistakes, pls do correct me :/ must appreciate this graph, okay! cause i took a million years to draw! :D but any mistakes, pls do correct me :/one example is ice melting - So how does a soild melt? Molecules in a soild are held by strong intermolecular bonds. Thermal energy is supplied to the soild, breaking the intermolecular bonds, allowing the molecules to move out of their fixed positions. Solidification & Freezing point - The reverse process of melting ( changing from a liquid to solid ) - Freezing point is the point whereby the liquid freezes to becomes a solid - -  Condensation Condensation- Is the change of state from vapour to liquid when it is cooled at the same temperature as boiling. - Thermal energy is given out  one example is the morning dew! :D one example is the morning dew! :D  Boiling - When a liquid is heated & changes to a gas at a fix temperature is called boiling. & that particular temperature is called its boiling point - Reverse of boiling is condensation  Evaporation - Evaporation can occur at any temperature  Apllications of evaporation Apllications of evaporation1) Cooling effect when any liquid evaporates from your skin 2) A puddle of water dries up after some time 3) Whenever you're sick, your mama will put a wet cloth on your forehead & when the water evaporate, there will be a cooling effect, keeping temperature down. Factors affecting the rate of evaporation 1) Temperature The higher the surrounding temperature, the higher the rate of evaporation. 2) Humidity of the surrounding air Rate of evaporation increases with decreasing humidity. 3) Surface area of liquid More surface area exposed to the air, rate of evaporation will increase. 4) Movement of air Moving air increases the rate of evaporation. 5) Pressure Reducing the atmospheric pressure increases the rate of evaporation 6) Boiling point of the liquid Liquids with lower boiling points will evaporate faster. Wednesday, September 3, 2008
Chapter 8.5 - Applications of Thermal Energy TransferUses of good conductors of heat 1) Cooking Utensils  Imagine, your cooking pot is made out of wood/plastic, its gonna take a billion years for your food to get cooked. That is why our cooking pots, saucepans are made out of metal, this is to ensure direct heating is involved. Imagine, your cooking pot is made out of wood/plastic, its gonna take a billion years for your food to get cooked. That is why our cooking pots, saucepans are made out of metal, this is to ensure direct heating is involved.2) Soldering iron rods  the tip is made out of copper as copper is a better conductor of heat then iron. 3) Heat exchangers helps save energy, mostly used in large laundry facility. Uses of bad conductors of heat (aka insulators) 1) Handles of appliances & utensils  do you notice that your cooking pots, saucepans, kettles have handles that are made out of plastic? this is because plastic are insulators & it will prevent us from burning our hands! :D do you notice that your cooking pots, saucepans, kettles have handles that are made out of plastic? this is because plastic are insulators & it will prevent us from burning our hands! :D2) Table mats  they are made out of cork so that your super hot kitchenware can be placed on the table mats without damaging your beautiful table. they are made out of cork so that your super hot kitchenware can be placed on the table mats without damaging your beautiful table.3) wooden ladles  extremely useful for stirring your soup or scooping up rice. extremely useful for stirring your soup or scooping up rice.Common applications of convection i remember we learnt something from geography about convection! 1) Convection currents in the mantle  remember to click to enlarge! & paiseh, i scan until like that. hhahas remember to click to enlarge! & paiseh, i scan until like that. hhahas&&&&& 2) Sea & Land Breezes  YAY! physics & geography can relate! :D :D :D YAY! physics & geography can relate! :D :D :D3) Radiator   Common applications of radiation 1) Teapot  have you ever wondered why is your teapot always made out of shiny material & not dark materials? remember the 3 factors of the rate of infrared radiation? As shiny surfaces are bad absorbers of radiation, it can keep your drinks hot longer then dark surfaces (: have you ever wondered why is your teapot always made out of shiny material & not dark materials? remember the 3 factors of the rate of infrared radiation? As shiny surfaces are bad absorbers of radiation, it can keep your drinks hot longer then dark surfaces (:2) Greenhouse  infrared radiation passes through the glass roof of the greenhouse & this warms up the plants & soil. When these plants & soil gets warm, they emits infrared radiation too. however, this infrared radiation is unable to pass through the glass roof as it is different from the sun's infrared radiation. the amt of infrared radiation will slowly build up, causing the temperature to rise in the greenhouse. infrared radiation passes through the glass roof of the greenhouse & this warms up the plants & soil. When these plants & soil gets warm, they emits infrared radiation too. however, this infrared radiation is unable to pass through the glass roof as it is different from the sun's infrared radiation. the amt of infrared radiation will slowly build up, causing the temperature to rise in the greenhouse.3) Vacuum flasks made to minimise heat loss in 4 ways, conduction, convection, radiation & evaporation.  Stopper - is usually made out of poor conductors of heat. Stopper - is usually made out of poor conductors of heat.Vacuum - conduction & convection is prevented by the vacuum Silvered surface - to minimise heat loss thru radiation & it is able to reflect the radiant heat heat to the liquid, keeping the liquid warm. Tuesday, September 2, 2008
Chapter 8 - Transfer of Thermal Energy!Thermal energy always flows from a region of higher temperature to a region of lower temperature! Thermal engery can be transfered by these processes: Conduction, Convection & Radiaton! (aka CCR) heres a picture to summarise what is CCR!  1) Conduction Conduction is the process of thermal energy transfer without any flow of the material medium. conduction race! :D therefore, we can conclude 2 important points: -Thermal energy flows through the materal of each rod without any flow of the material itself. -Different material conduct heat at different rates. Copper is good conductor of heat while steel is a poor conductor of heat (aka insulator) so, how does conduction work? When a rod is heated at one end, the particles of the hot end will vibrate vigorously, colliding with neighbouring particles, making them vibrate as well. Therefore, kinetic energy of the vibrating particles at the hot end is transferred to the neighbouring particles. JAVA SCRIPT --> (The one on the left is the conduction example) CLICK HERE! :D note that the particles at the hot end vibrating vigorously while the particles at the cooler ends vibrating less vigorously (: 2) Convection  convection is the transfer of thermal energy by means of currents in a fluid (liquid/gas) convection is the transfer of thermal energy by means of currents in a fluid (liquid/gas)convection in liquid convection in air  (actually i found one almost the same as the experiment to show convection in air, but the experiment doesnt seem to work, take a look if you want --> HERE! :l) SOOO, how does convection work? In water - when water is heated, it expands. Expanded water is less dense than the surrounding water, therefore it rises while the cooler water is denser, sinks. In air - The heated air expands & becomes less dense, rises, while the surrounding air, being denser, sinks. JAVA SCRIPT ON CONVECTION IN AIR ---> CLICK HERE! :D (its the one on the right) 3) Radiation radiation is the continual emission of infrared waves from the surface of all bodies, transmitted without the aid of a medium. *note! unlike conduction & convection, it does not require any medium for energy transfer! -Infrared radiation is absorbed by all objects & surface, this cause a temperation rise. Factors affecting rate of infrared radiation: 1) Colour & texture of the surface Black surface absorbs & emits infrared radiation better then shiny, white surfaces. 2)Surface temperature The higher the temperature of the surface of the object relative to the surrounding temperation, the higher the rate of infrared radiation. 3) Surface area The greater the surface area, it will emit infrared radiation at a higher rate. To be continued with the applications of thermal energy transfer... (: Monday, September 1, 2008
Chapter 7 - Kinetic Model or MatterSolid Properties: -Fixed shape & volume -Hard & Rigid -High Density -Incompressible 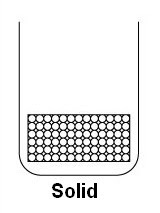 -Particles are closely packed in a regular pattern, occupying minimum space resulting in solids having high densities. -Particles are closely packed in a regular pattern, occupying minimum space resulting in solids having high densities.-Particles also vibrate about fixed positions as they are held in position by very strong intermolecular bonds. This is why solids have a fixed volume and shape. Liquid Properties: -Fixed volume but does not have a fixed shape -High density -Incompressible  -Particles of liquid are randomly arranged with particles slightly further apart then solids, therefore, this results in liquid having relatively high densities. -Particles of liquid are randomly arranged with particles slightly further apart then solids, therefore, this results in liquid having relatively high densities.-The particles are free to move about but is confined within the vessel containing it & have attractive forces between the particles. Therefore liquids have fixed volumes but no fixed shape. Gas Properties: -No fixed shape & volume -Low density -Compressible  -Particles are very far apart & are randomly arranged, occupying any available space, therefore, gases have very low density -Particles are very far apart & are randomly arranged, occupying any available space, therefore, gases have very low density-The particles of gas have very little attraction between them & move about randomly at very high speed. This explains why gases have no fixed volume, shape and is compressible. Brownian motion -is the random/irregular motion of smoke particles in air -only occurs in fluids (any substance that is able to flow/has particles that can move freely) -Higher the temperature, the motion of smoke particles will be come more vigorous. Therefore, we can conclude that air molecules have greater speed at higher temperatures. java applet -->Brownian Motion Wednesday, August 27, 2008
YAY! BLOG DONE! :Dthermalphysics;thermalphysics :l |